Hydrogen, in its purest form, is a colorless, odorless, and non-toxic gas. These properties might lead some to assume that it poses no significant risk to the environment. But when we consider its tendency to escape or “fugitively” emit into the atmosphere, we realize the potential consequences it can have on our environment.
Fugitive emissions occur when hydrogen escapes from production, transportation, or storage systems through leaks, venting, purging, or other unintended pathways. Unlike carbon dioxide, which is a heavier gas and tends to disperse closer to the ground, hydrogen is lighter and can rapidly rise and spread. A comparison of greenhouse gases molecular weights and hydrogen is shown in Table 1.
Table 1: Gases vs Molecular Weight
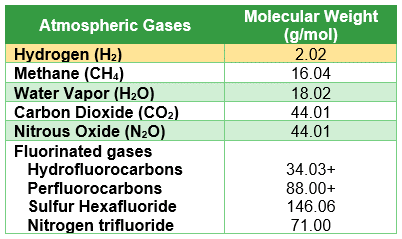
Table1: The table shows five common types of greenhouse gases (methane, water vapor, carbon dioxide, nitrous oxide, and fluorinated gases) and their molecular weights compared to the molecular weight of hydrogen.
Hydrogen Fugitive Emissions and Global Warming
Hydrogen takes two primary routes when emitted to the atmosphere.
- Approximately 70-80% of the hydrogen is estimated to be removed by soils via diffusion or bacterial uptake.
- The remaining 20-30% is oxidized by reacting with the naturally occurring hydroxyl radical (OH) groups. The oxidation of hydrogen in the atmosphere leads to increasing concentrations of greenhouse gases in both the troposphere and stratosphere.[1]
Understanding Global Warming – Methane and Carbon Cycles
In the methane cycle, the primary method for removing methane is to react it in the troposphere with the hydroxyl radical (OH). Hydroxyl radicals are considered “sinks” because they scrub the atmosphere of pollutant molecules (i.e., methane) and break them down. After methane reacts with an OH group, it is converted to CO2 by a series of long chemical reactions. Some of the methane present in the troposphere passes into the stratosphere where the same process scrubs the atmosphere clean of methane.
In the carbon cycle, the CO2 from the atmosphere exchanges with ocean life (phytoplankton) and eventually the carbon is returned to the earth’s crust.
Human activities have “short-circuited” these natural cycles. Emissions of greenhouse gases are reaching the atmosphere at concentrations greater than the natural cycle creating a warming effect. In addition to methane and carbon dioxide, other primary greenhouse gases are methane, carbon dioxide, nitrous oxide, water vapor, and fluorinated gases. To allow the comparison of the global warming impacts of different gases compared to those of CO2, the Global Warming Potential (GWP) was developed.
Research suggests that hydrogen fugitive emissions also result in an “indirect warming effect” because it contributes to reactions that propagate warming. This is further described in Figure 1.
- Hydrogen and methane will both react with hydroxyl groups in the troposphere. As a result of the competition for hydroxyl groups, some methane will last longer allowing it to contribute to warming effects for a longer period.
- In the troposphere hydrogen can undergo a chain of reactions to create ozone molecules (O3), another greenhouse gas.
In the stratosphere, the hydrogen will react with hydroxyl groups and increase the water vapor content. This increases the infrared radiative capacity of the stratosphere; meaning that more heat is re-radiated to earth’s surface causing warming.
Figure 1: Global Warming Effects of Hydrogen Emissions
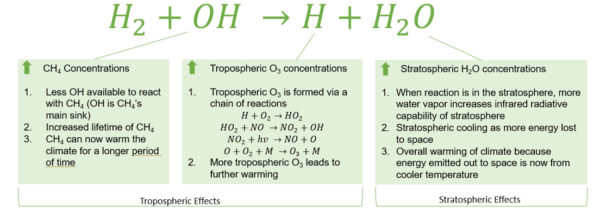
Figure 1: Effects of hydrogen oxidation on atmospheric greenhouse gas concentrations and warming [1].
Scientists and researchers attempt to quantify the impact of hydrogen fugitive emissions on global warming today and in the future. Several assumptions are made in these estimates and therefore projections vary especially when comparing the 20-year vs 100-year global warming potential (GWP) horizons. Some suggest that hydrogen’s warming potential is estimated to be 40 times greater than that of CO2 in the first 20 years after it is released [2]. Others say that hydrogen’s warming potency may be 3 times greater than CO2 in the near term [1]. Some of the questions and assumptions include:
- How much hydrogen will be produced in the future? Will this offset other greenhouse gas emissions?
- How much hydrogen leaks from hydrogen systems (production, transportation, storage)?
- Does the technology utilized, (i.e., gray vs green hydrogen production) impact leakage calculations?
- What type of leak detection technology is available to find small leaks? Will they be addressed?
- How is the hydrogen warming impact calculated?
- What is an acceptable leak rate for hydrogen?
Managing Hydrogen Fugitive Emissions
Proper handling, storage, and transportation infrastructure must be in place to mitigate the risks associated with potential leaks of hydrogen for both safety and environmental factors. There are several strategies that could be implemented.
- Ensure minimized leaks through proper sealing solutions across the hydrogen value chain.
- Implement stringent safety protocols and leak detection technologies that can help identify and eliminate fugitive emissions.
Both approaches ensure safe use and deployment of hydrogen in a net zero emission economy.
Hydrogen Sealing Solutions
Today hydrogen is predominantly produced from steam methane reforming (SMR), known as “gray” hydrogen. Even in cleaner methods of hydrogen production, such as electrolysis using renewable energy (green hydrogen), fugitive hydrogen emissions can still occur.
To combat hydrogen leaks pre-emptively, Flexitallic can offer solutions utilizing Corriculite® and Thermiculite® for hydrogen sealing . These products are proven to seal with leak rates 100-1000x tighter than conventional solutions, minimizing leak rates. Figure 2 shows a logarithmic scale of leak rate vs gasket stress of different materials during an EN13555 test method.
EN13555 Test Method
Characterizes a gasket material’s ability to seal and maintain a seal when exposed to a standardized regime of compressive loading and unloading. The lower and flatter the residual assembly stress the better the material’s capability of keeping a seal due to unloading. We know that flange joints can become unloaded for a variety of reasons in operation; the lower the residual stress the greater the sealing capacity and the safer the joint will be.
Though the data represented shows the entire EN13555 test results, it is important to note that Flexitallic recommends a minimum sealing stress of 68 MPa for spiral wound gaskets. The gasket stress 68 MPa and above is what would be seen in operation. Corriculite® and Thermiculite® seal at orders of magnitude lower (i.e., much lower leak rates) than a standard graphite material. Best in class technology can minimize hydrogen emissions.
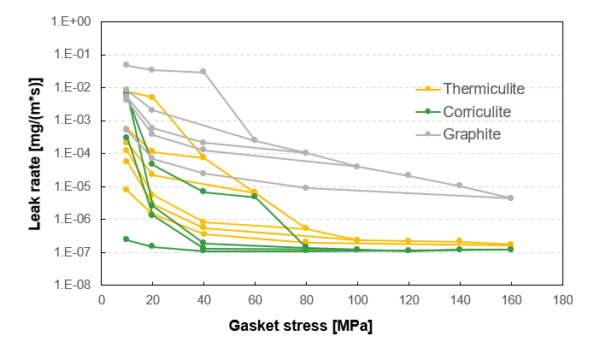
Figure 2: EN13555 test results of spiral wound gaskets with filler materials composed of graphite, Corriculite®, and Thermiculite® using hydrogen testing at 40 bar and 20°C.
Fugitive Hydrogen Monitoring Solutions
The unintended consequence of fugitive hydrogen emissions also highlights the importance of preventing hydrogen leaks and developing effective monitoring systems.
To address the risk of hydrogen leaks and fugitive emissions, robust and standardized leak detection technologies should be utilized. Continuous monitoring systems that utilize advanced sensors, such as infrared cameras, acoustic detection, or laser-based detectors, can quickly identify leaks and allow for prompt repairs. Early detection not only helps prevent environmental damage but also ensures the efficient use of hydrogen, minimizing losses while maintaining safe operating environments.
Conclusion
While hydrogen fugitive emissions pose challenges, we should not lose sight of the overall benefits that hydrogen offers. It has the potential to play a significant role in decarbonizing sectors such as transportation, industry, and power generation. By leveraging hydrogen as an energy carrier, we can reduce greenhouse gas emissions and move towards a cleaner, more sustainable future.
As we embrace the potential of hydrogen as a clean energy source, we must acknowledge the challenges posed by fugitive emissions and the risk of leaks to the environment. Implementing robust sealing solutions, leak detection technologies, and investing in research and development are crucial steps towards mitigating these risks. By addressing these challenges head-on, we can maximize the benefits of hydrogen and pave the way for a greener and more sustainable future.
References
[1] | I. B. Ocko and S. P. Hamburg, “Climate consequences of hydrogen emissions,” European Geosciences Union, vol. 22, no. 14, pp. 9349-9368, 2022. |
[2] | S. Menon, “New Technology can catch hydrogen leaks, protect climate as industry booms,” 7 March 2023. [Online]. Available: https://www.edf.org/article/new-technology-can-catch-hydrogen-leaks-protect-climate-industry-booms. |
[3] | R. M. K. R. A. W. a. T. W. Thomas Koch blank, “Hydrogen Reality Check #1: Hydrogen Is Not a Significant Warming Risk,” Rocky Mountain Institute, 9 May 2022. [Online]. Available: https://rmi.org/hydrogen-reality-check-1-hydrogen-is-not-a-significant-warming-risk/. [Accessed 12 June 2023]. |
[4] | United States Environmental Protection Agency, “Understanding Global Warming Potentials,” United States Environmental Protection Agency, 18 April 2023. [Online]. Available: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials#:~:text=Just%20like%20the%20100%2Dyear,years%20after%20the%20emissions%20occur.. [Accessed 19 July 2023]. |
[5] | M. J. Maple, “Is Hydrogen a Greenhouse Gas?,” DNV, 11 May 2023. [Online]. Available: https://www.dnv.com/article/is-hydrogen-a-greenhouse-gas–243214. [Accessed 19 July 2023]. |
Author: Shannon McAvoy; November 28, 2023

